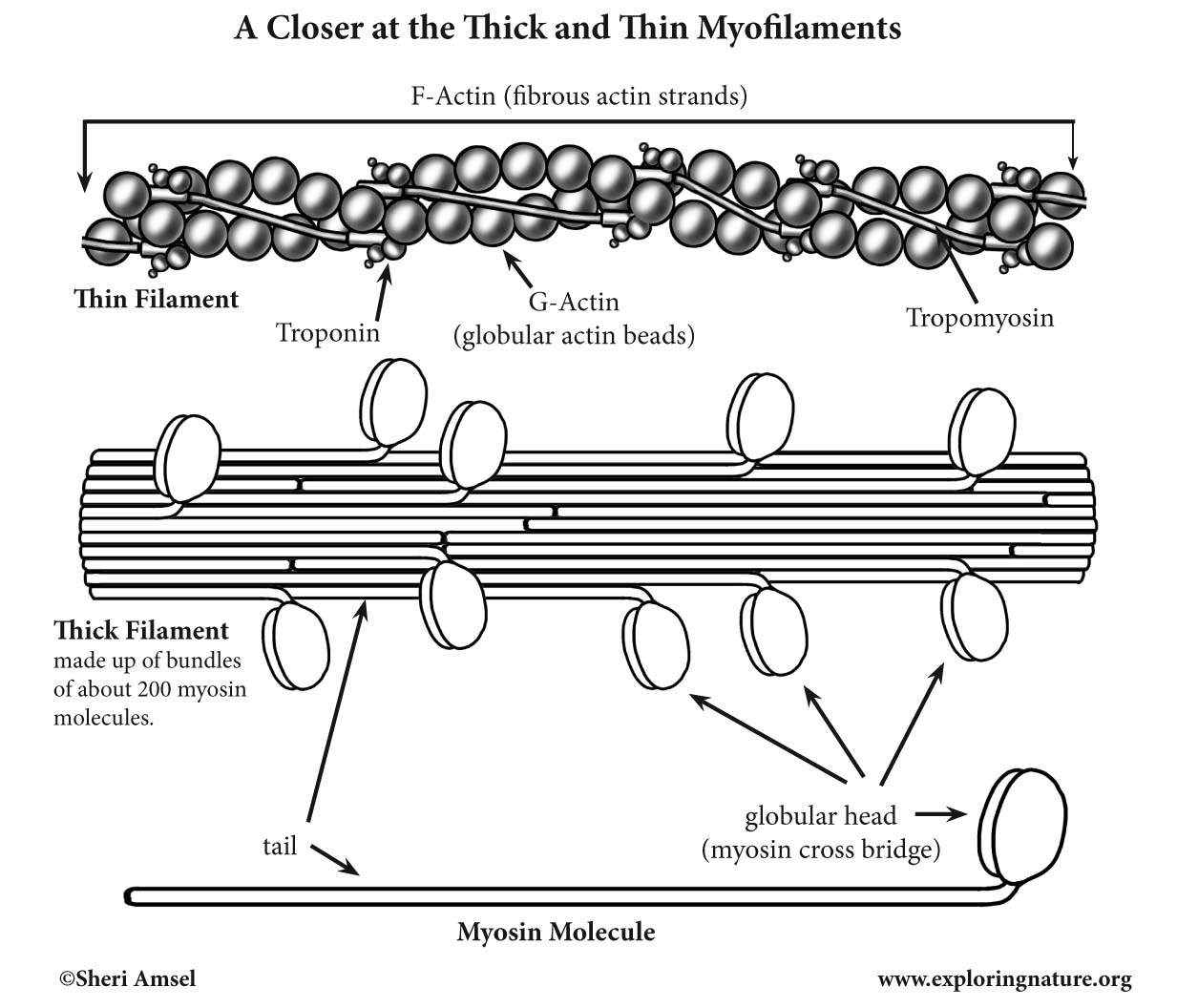
Thick filaments are made of about 200 Myosin molecules (contractile proteins) bundled together.
Each Myosin molecule has a tail and two globular heads (or myosin cross bridges). The Myosin heads contain ATPases (to split ATP) and ATP binding sites.
Thin filaments are made up of Actin. G-actin (globular actin) are active sites to which the myosin cross-bridges can bind during muscle contraction.
Long strands of the G-actin beads form F-actin (fibrous actin) strands. The backbone of each thin filament is two of these strands of F-actin coiled around each other.
Each thin filament is strengthened by a stiff protein, called Tropomyosin, which spirals around each F-Actin core. There is also another protein, called Troponin, made up of three polypeptides: one binds to actin, one binds to tropomyosin and one binds to Ca+ (calcium ions). These two proteins help to control the cross bridging of myocin and actin during muscle contractions.
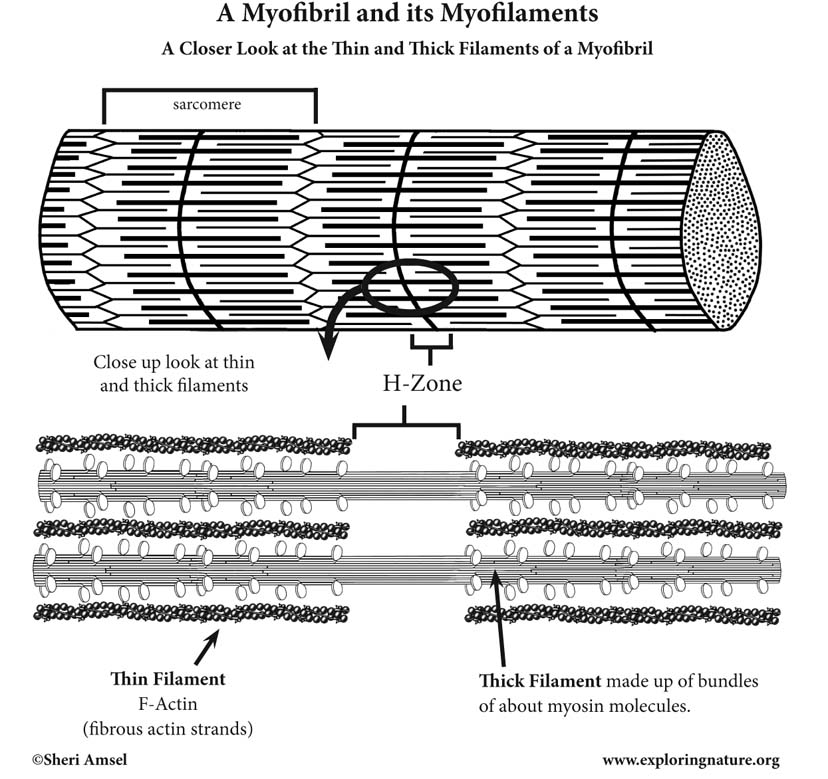
Closer Look at the Thin and Thick Filaments of a Myofibril
Where the thin and thick filaments over lap, the thick filaments have globular heads (myosin cross bridges) that, during muscle contraction, will grab onto the G-Actin sites on the thin filaments and pull them toward the center of the sarcomere, shortening them.
When muscles are relaxed, the center of a sarcomere has a gap in the thin filaments – called the H-Zone. Here the thick filaments have no globular heads (myosin cross bridges), because there are no G-Actin sites to grab onto during contractions.