Disciplinary Core Ideas
LS1.C: Organization for Matter and Energy Flow in Organisms
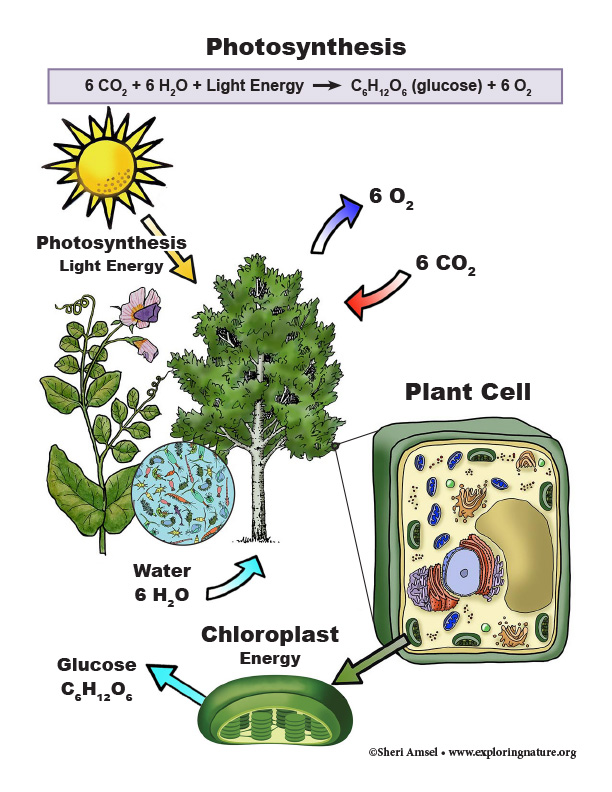
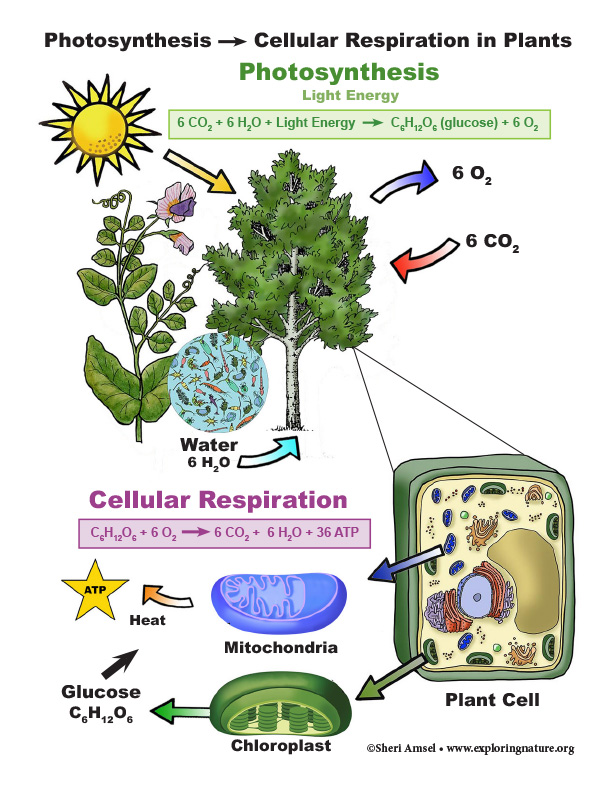
• The process of photosynthesis converts light energy to stored chemical energy by converting carbon dioxide plus water into sugars plus released oxygen. (HS-LS1-5)
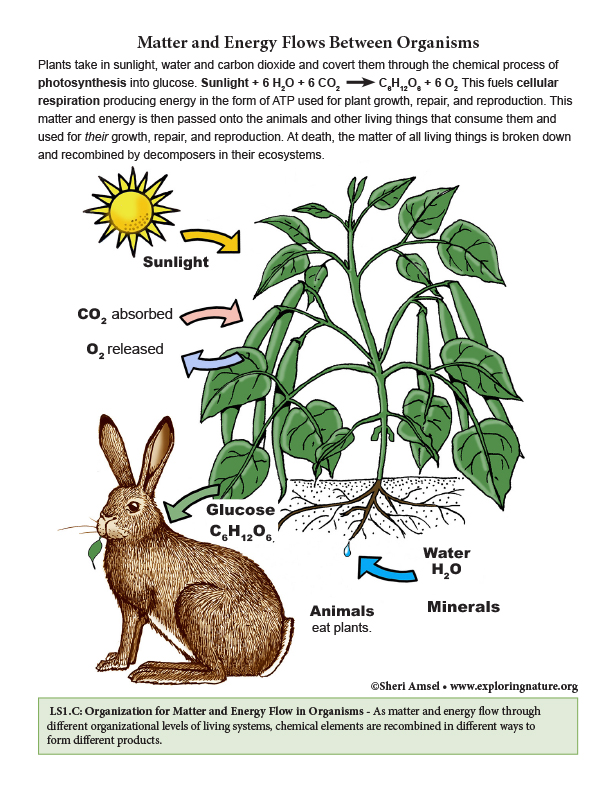
• The sugar molecules thus formed contain carbon, hydrogen, and oxygen: their hydrocarbon backbones are used to make amino acids and other carbon-based molecules that can be assembled into larger molecules (such as proteins or DNA), used for example to form new cells. (HS-LS1-6)
• As matter and energy flow through different organizational levels of living systems, chemical elements are recombined in different ways to form different products. (HS-LS1-6),(HS-LS1-7)
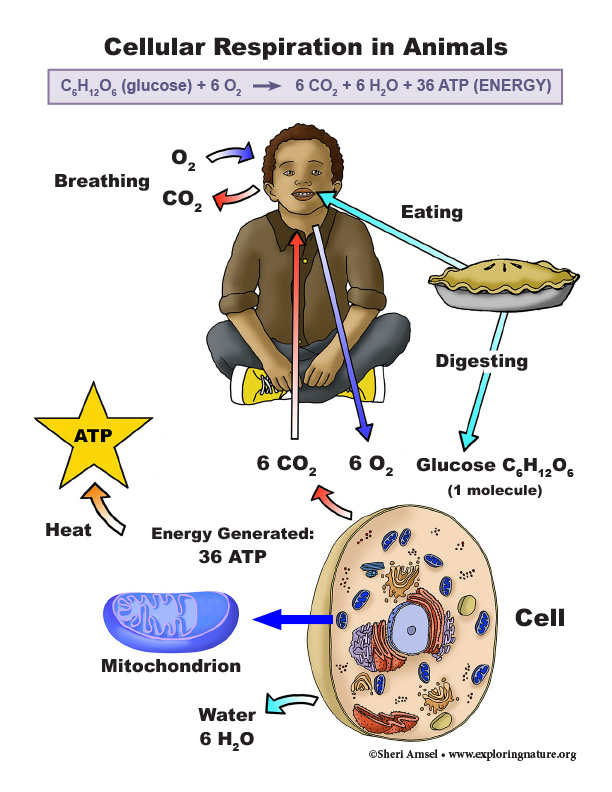
• As a result of these chemical reactions, energy is transferred from one system of interacting molecules to another. Cellular respiration is a chemical process in which the bonds of food molecules and oxygen molecules are broken and new compounds are formed that can transport energy to muscles. Cellular respiration also releases the energy needed to maintain body temperature despite ongoing energy transfer to the surrounding environment. (HS-LS1-7)
Use the Template and Resource Links to Fulfill NGSS
l. Goals:
Essential Questions:
NGSS Note: Think, question, entertain ideas.
ll. Introductory Activities to Assess Prior Knowledge
A. Simple Activities - that assess students’ understanding of photosynthesis and cellular respiration.
Photosynthesis and Cellular Respiration Model Labeling
Photosynthesis Short Answer Quiz
Cellular Respiration in Animal Short Answer Quiz
lll. New Knowledge - Text
A. Read about photosynthesis and cellular respiration:Photosynthesis and Cellular Respiration
Matter and Energy Flows Between Organisms
B. Examples of Models (depicts the concept expressed in the reading):
Ask students to look at the models and explain how each illustrates the concepts they've read about.
Cellular Respiration - Authentic Performance Activity
Sunlight Crisis - Volcanic Apocalypse Authentic Performance
V. Summarize Knowledge - Enduring Understandings
Vl. Next Generation of Science Standards (NGSS) - High School Life Science
Disciplinary Core Ideas
LS1.C: Organization for Matter and Energy Flow in Organisms
• The process of photosynthesis converts light energy to stored chemical energy by converting carbon dioxide plus water into sugars plus released oxygen. (HS-LS1-5)
• The sugar molecules thus formed contain carbon, hydrogen, and oxygen: their hydrocarbon backbones are used to make amino acids and other carbon-based molecules that can be assembled into larger molecules (such as proteins or DNA), used for example to form new cells. (HS-LS1-6)
• As matter and energy flow through different organizational levels of living systems, chemical elements are recombined in different ways to form different products. (HS-LS1-6),(HS-LS1-7)
• As a result of these chemical reactions, energy is transferred from one system of interacting molecules to another. Cellular respiration is a chemical process in which the bonds of food molecules and oxygen molecules are broken and new compounds are formed that can transport energy to muscles. Cellular respiration also releases the energy needed to maintain body temperature despite ongoing energy transfer to the surrounding environment. (HS-LS1-7)
Science and Engineering Practices
Developing and Using Models
Modeling in 9–12 builds on K–8 experiences and progresses to using, synthesizing, and developing models to predict and show relationships among variables between systems and their components in the natural and designed worlds.
• Develop and use a model based on evidence to illustrate the relationships between systems or between components of a system. (HS-LS1-2)
• Use a model based on evidence to illustrate the relationships between systems or between components of a system. (HS-LS1-4),(HS-LS1-5),(HS-LS1-7)
Constructing Explanations and Designing Solutions
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories.
• Construct an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future. (HS-LS1-1)
• Construct and revise an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future. (HS-LS1-6)
Crosscutting Concepts
Systems and System Models
• Models (e.g., physical, mathematical, computer models) can be used to simulate systems and interactions—including energy, matter, and information flows—within and between systems at different scales. (HS-LS1-2),(HS-LS1-4)
Energy and Matter
• Changes of energy and matter in a system can be described in terms of energy and matter flows into, out of, and within that system. (HS-LS1-5), (HS-LS1-6)
• Energy cannot be created or destroyed—it only moves between one place and another place, between objects and/or fields, or between systems. (HS-LS1-7
Performance Expectations
Students who demonstrate understanding can:
HS-LS1-5. Use a model to illustrate how photosynthesis transforms light energy into stored chemical energy. [Clarification Statement: Emphasis is on illustrating inputs and outputs of matter and the transfer and transformation of energy in photosynthesis by plants and other photosynthesizing organisms. Examples of models could include diagrams, chemical equations, and conceptual models.] [Assessment Boundary: Assessment does not include specific biochemical steps.]
HS-LS1-6. Construct and revise an explanation based on evidence for how carbon, hydrogen, and oxygen from sugar molecules may combine with other elements to form amino acids and/or other large carbon-based molecules. [Clarification Statement: Emphasis is on using evidence from models and simulations to support explanations.] [Assessment Boundary: Assessment does not include the details of the specific chemical reactions or identification of macromolecules.]
HS-LS1-7. Use a model to illustrate that cellular respiration is a chemical process whereby the bonds of food molecules and oxygen molecules are broken and the bonds in new compounds are formed resulting in a net transfer of energy. [Clarification Statement: Emphasis is on the conceptual understanding of the inputs and outputs of the process of cellular respiration.] [Assessment Boundary: Assessment should not include identification of the steps or specific processes involved in cellular respiration.]
When you research information you must cite the reference. Citing for websites is different from citing from books, magazines and periodicals. The style of citing shown here is from the MLA Style Citations (Modern Language Association).
When citing a WEBSITE the general format is as follows.
Author Last Name, First Name(s). "Title: Subtitle of Part of Web Page, if appropriate." Title: Subtitle: Section of Page if appropriate. Sponsoring/Publishing Agency, If Given. Additional significant descriptive information. Date of Electronic Publication or other Date, such as Last Updated. Day Month Year of access < URL >.
Amsel, Sheri. "LS1.C: Organization for Matter and Energy Flow in Organisms (HS-LS1 From Molecules to Organisms: Structures and Processes)" Exploring Nature Educational Resource ©2005-2024. December 13, 2024
< http://www.exploringnature.org/db/view/LS1C-Organization-for-Matter-and-Energy-Flow-in-Organisms-HS-LS1-From-Molecules-to-Organisms-Structures-and-Processes >