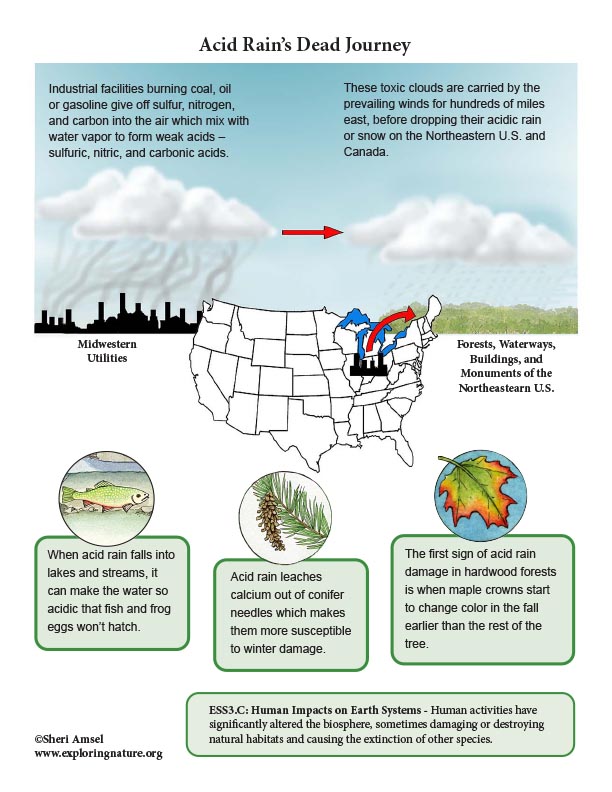
Acid Rain is created when large industrial facilities (e.g. Midwestern utilities) burns coal or oil which gives off sulfur, nitrogen, and carbon into the air. Their smokestacks carry these waste gas emissions into the clouds where they mix with water vapor to form weak acids – sulfuric, nitric, and carbonic acids. The wind patterns of North America carry pollutants from busy cities, factories and industrial centers in the Midwest for hundreds of miles east to the Northeastern U.S. and Canada. When the moisture in the clouds grows heavy enough, it falls as rain - acid rain (or snow). The forests and communities of the northeastern U.S. and Canada have been the victim of acid rain that started as air pollution from Midwestern utilities for the last 100 years.
The effects of Acid Rain:
• When acid rain falls into lakes and streams, it can make the water so acidic that fish and frog eggs won’t hatch.
• It corrodes limestone buildings, walkways, and statues. Acid rain can slow the growth of trees.
• It leaches calcium out of conifer needles which makes them more susceptible to winter damage. It also leaches the calcium and magnesium from the soil. Calcium and magnesium are important for the health of the forest because these components are “base cations.” They act as natural buffers in the soil, keeping it from being too acidic. Once they are depleted, the aluminum in the soil dissolves and can be absorbed by trees and plants and acts as a toxin that damages them. In sugar maples, the damage starts to show up as a die off in the top of the crown of the tree. The first sign of acid rain damage to a hardwood forest is when maple crowns start to change color in the fall earlier than the rest of the tree.
Much has been done to decrease acid rain in the last few decades. In 1990, congress passed Clean Air Act Amendments which require companies to reduce their sulfur emissions. One way they do this is by installing “scrubbers” in their smokestacks. They release a liquid alkaline that mixes with the emissions and traps 80-95% of the sulfur pollutants before it can escape into the air. But soils take thousands of years to develop so recovery is very, very slow.
Performance Expectations - Students who demonstrate understanding can:
MS-LS1-5. Construct a scientific explanation based on evidence for how environmental and genetic factors influence the growth of organisms.
When you research information you must cite the reference. Citing for websites is different from citing from books, magazines and periodicals. The style of citing shown here is from the MLA Style Citations (Modern Language Association).
When citing a WEBSITE the general format is as follows.
Author Last Name, First Name(s). "Title: Subtitle of Part of Web Page, if appropriate." Title: Subtitle: Section of Page if appropriate. Sponsoring/Publishing Agency, If Given. Additional significant descriptive information. Date of Electronic Publication or other Date, such as Last Updated. Day Month Year of access < URL >.
Amsel, Sheri. "Acid Rain - How Environmental Factors Influence the Growth of Organisms (NGSS 6-8th Grade)" Exploring Nature Educational Resource ©2005-2024. December 14, 2024
< http://www.exploringnature.org/db/view/Acid-Rain-How-Environmental-Factors-Influence-the-Growth-of-Organisms-NGSS-6-8th-Grade >